This is a catelogy that included many hot sale kinds of car accessory.After careful analysis, we put all kinds of popular products into this category.The products in this catelogy are all very hot sale and we prepare for to help you more quickly to find which is your really want.You can see all kinds of products here. If you take them home, they will definitely help you increase sales. Car Humidifier,Car Charger,Car Fan Ningbo Autrends International Trade Co.,Ltd. , https://www.nbcaraccessories.com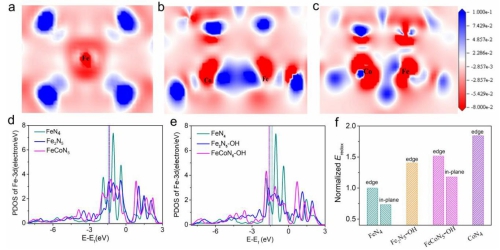
Advances in research on diatomic catalytic centers for simultaneous control of electronic structure and space configuration
[ Instrument Network Instrument R & D ] Monoatomic MNC materials are considered to be the most promising non-noble metal ORR catalysts, and their active centers have been identified as metal-nitrogen coordination structures that mimic biological porphyrin centers. Previous research work has shown that Metal-centered MNC catalysts follow the Sabatier principle, where the "exact" MO bond strength helps maximize catalytic performance. The Fe-NC catalyst is located at the right end of the MNC volcanic curve. The binding energy is too strong and there is still a distance from the apex. Fe (III) / Fe (II) redox potential (Eredox) can be used as an effective indicator of MO binding energy. Increasing Eredox will weaken the Fe-O binding energy and promote the performance of Fe-based catalysts to the top of the volcanic curve.
Based on this, Xing Wei and Ge Junjie, researchers at the Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, and Chen Shengli, a professor at Wuhan University, and Jiang Zheng, a researcher at Shanghai Light Source, designed the FeCoN5 diatomic site. Water at this center spontaneously dissociates to generate new FeCoN5OH stable sites. The d-orbital energy level of Fe can be adjusted to increase the Eredox of Fe.
Combining DFT theoretical calculations with in-situ X-ray near-side absorption spectroscopy (XANES) confirms that the Fe (III) / Fe (II) redox potential at the new active site is improved, thereby significantly improving ORR performance and promoting Fe-based catalytic The top of the volcano curve moves. Experiments have confirmed that the FeCoN5-OH site is extremely active, exhibiting approximately 20 times the intrinsic activity of the FeN4 site, and has unprecedented ORR activity in acid electrolytes (Eonset = 1.02 V, E1 / 2 = 0.86 V). Due to tailor-made electronics and space geometry.
It can be expected that the combination of the OH-ligand strategy with the clustered metal center structure can develop more efficient catalysts with higher ORR activity, thus making the substitution of Pt-based catalysts truly feasible. This discovery opens up new ideas for modulating the electronic structure of active sites at the atomic level, and provides new insights into the comprehensive understanding of the diatomic active centers of ORR electrocatalysts. This article was published in the Journal of the American Chemical Society (J. Am. Chem. Soc., 2019, 141, 17763-17770).